UniColl® for the most stringent safety & performance requirements
Specially produced for medical applications and research
Our UniColl® native collagen meets even the most stringent requirements in terms of safety and quality. Relying on state-of-the-art clean-room production and advanced technologies, we offer a product that convinces due to its purity, biocompatibility and versatile application possibilities. With UniColl® you take your medical and scientific products to the next level.

© KBM

© KBM
Origin & quality of the raw materials
Gentle extraction to retain natural properties
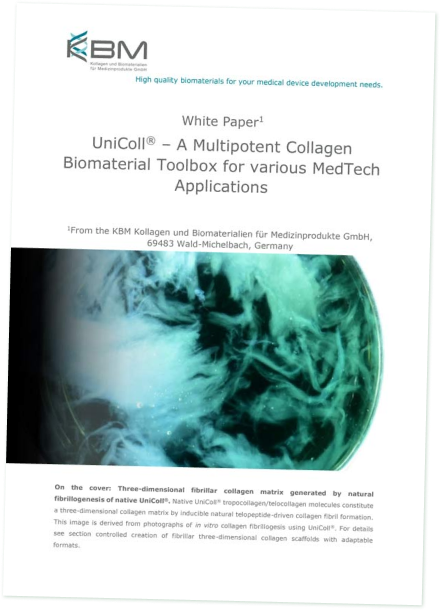
UniColl® is a medical collagen extracted from bovine hides. These come exclusively from countries with the lowest possible BSE risk, which corresponds to the high requirements of the ISO 22442 standard. Through the selection of premium quality raw materials, we ensure that UniColl® meets the most stringent safety standards. Using acidic extraction, we obtain pure type I collagen directly from the dermis of the bovine hide. The native collagen thus extracted has a triple helical conformation and contains both N- and C-terminal telopeptides (telocollagen), which retains its natural biological properties.
Endotoxin-free collagen
Production under clean-room conditions
UniColl® is manufactured in a clean-room production environment which meets the requirements of ISO 14644-1, Class 7. Under laminar-flow conditions, a proprietary method for the deactivation of endotoxins is used, which guarantees endotoxin-free (< 0.5 EU/mg) collagen. The modern production environment and the strict quality inspections make UniColl® a medical collagen of highest purity and quality. The proven endotoxin deactivation method is a decisive factor for the safety and tolerability of UniColl®.

© KBM

© KBM
Native collagen – This is how we assure quality
The entire UniColl® production process – ranging from the careful procurement of the raw materials up to clean-room production – is consistently aimed at quality and safety. Through strict inspections and innovative technologies, we ensure a collagen product that meets the highest standards.
- Procurement of raw materials to ISO 22442
- Extracted from bovine hides from countries not affected by BSE
- Production facilities of clean-room class 7 according to ISO 14644-1
- Endotoxin-free
- High biocompatibility demonstrated by in vivo implantation studies
For reliable results
Biological activity & a wide range of application possibilities
Thanks to its intact native structure, UniColl® retains its natural biological activities. This includes the supra-molecular self-assembly capacity of fibrils, cell-matrix interactions, biocompatibility and bio-remodelling. These properties make UniColl® a versatile technological building block for a great variety of applications in the development and manufacture of medical products as well as in biomedical research. Its top quality and compliance with the most stringent requirements ensure that UniColl® fully meets the safety & performance requirements for medical applications.

© KBM